Process of registration of Medical device
The process of registering a medical device in India can be complex and involves several steps. Here is a simplified overview of the typical registration process:
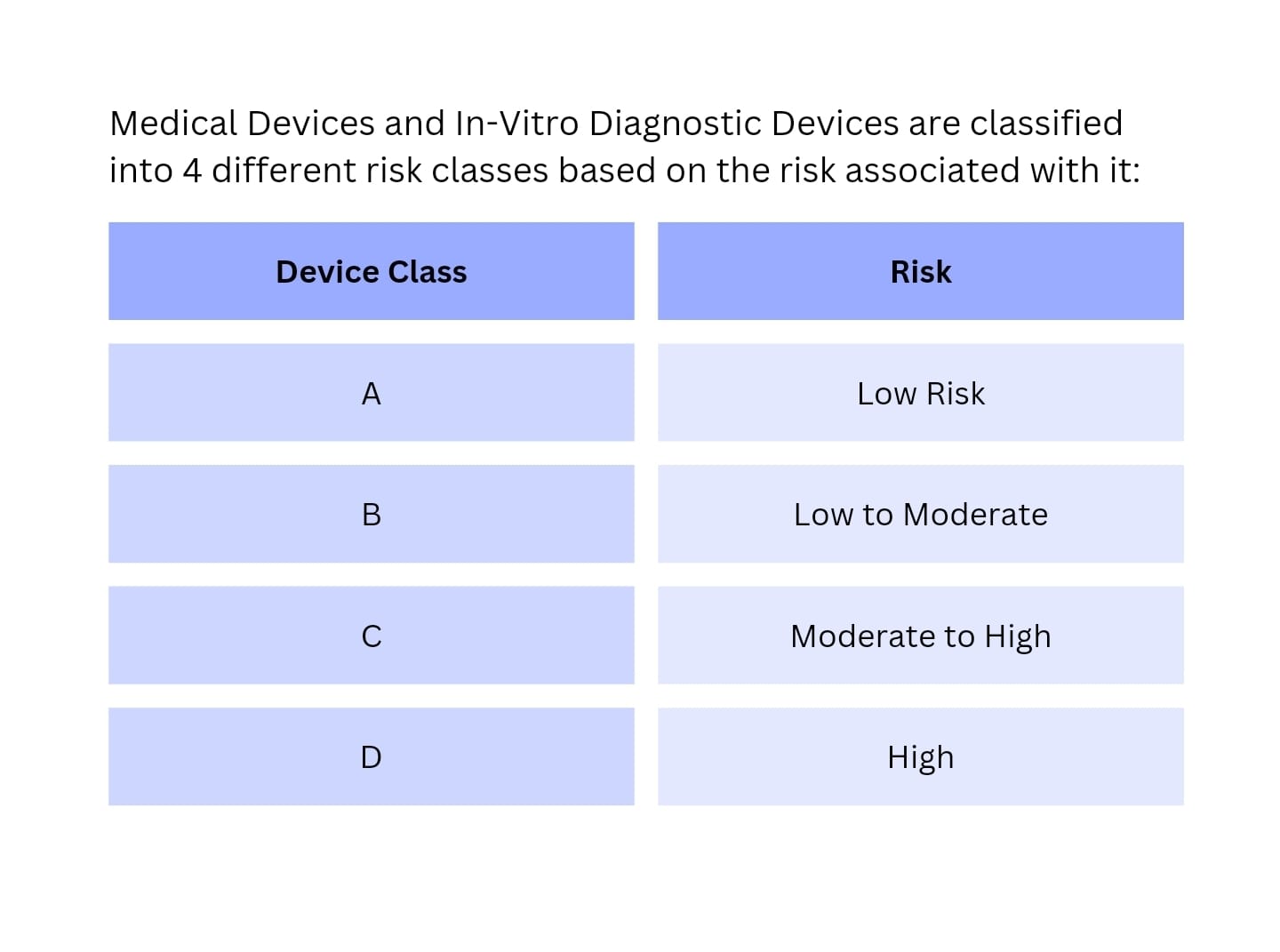
- Determine Device Classification: The first step is to determine the classification of your medical device according to the Indian regulatory framework. Devices are classified into different categories based on their risk and intended use.
- Appoint an Indian Agent: Foreign manufacturers are required to appoint an authorized Indian agent who will represent them before the Indian regulatory authorities.
- Prepare Documentation: Prepare a comprehensive set of documents, including:
- Device technical specifications and labeling
- Risk assessment report
- Clinical data (if applicable)
- Quality management system certification (e.g., ISO 13485)
- Declaration of conformity
- Submit Application: Submit your registration application along with the required documents to the Central Drugs Standard Control Organization (CDSCO). The application should be submitted through the Online System for Medical Devices (OSMD).
- Review and Evaluation: The CDSCO will review your application and documents. This may involve a thorough examination of your device's technical data and safety information.
- Clinical Trials (if necessary): Depending on the risk classification of your device, clinical trials may be required to establish safety and efficacy. This step is not applicable to all devices.
- Inspection of Manufacturing Site: The CDSCO may conduct an inspection of the manufacturing facility to ensure compliance with quality standards.
- Registration Certificate: If your application is approved, you will receive a Registration Certificate from the CDSCO. This certificate allows you to legally market and sell your medical device in India.
- Post-Market Surveillance: After registration, it's essential to establish a post-market surveillance system to monitor the safety and performance of your device and promptly report any adverse events.
- Renewal and Compliance: Medical device registrations typically need to be renewed periodically. Ensure ongoing compliance with evolving regulations and standards.
Please note that the specifics of the registration process may vary depending on the device's classification and other factors. It is crucial to consult with regulatory experts or a consultancy like Terz Compliance for precise guidance tailored to your device and regulatory requirements. Additionally, regulations and processes may change over time, so staying updated with the latest information from the CDSCO is essential.